published: October, 2022.
Years of research exploring mRNA vaccines for cancer treatment in preclinical and clinical trials have set the stage for the rapid development of mRNA vaccines during the COVID-19 pandemic. Therapeutic cancer vaccines based on mRNA are well tolerated, and the inherent advantage in ease of production, which rivals the best available conventional vaccine manufacture methods, renders mRNA vaccines a promising option for cancer immunotherapy.
Technological advances have optimised mRNA-based vaccine stability, structure, and delivery methods, and multiple clinical trials investigating mRNA vaccine therapy are now enrolling patients with various cancer diagnoses. Although therapeutic mRNA-based cancer vaccines have not yet been approved for standard treatment, encouraging results from early clinical trials with mRNA vaccines as monotherapy and in combination with checkpoint inhibitors have been obtained. This Review summarises the latest clinical advances in mRNA-based vaccines for cancer treatment and reflects on future perspectives and challenges for this new and promising treatment approach.
Introduction
The COVID-19 pandemic has directed worldwide focus towards mRNA-based vaccines. Indeed, the foundation for the rapid COVID-19 vaccine development and production was based on years of research exploring mRNA vaccines as a therapeutic strategy against cancer in preclinical and clinical trials.1
mRNA brings several benefits to a vaccine setting (panel). Firstly, mRNA-based vaccines are well tolerated, easily degraded, and do not integrate into the host genome. 2, 3 , 4
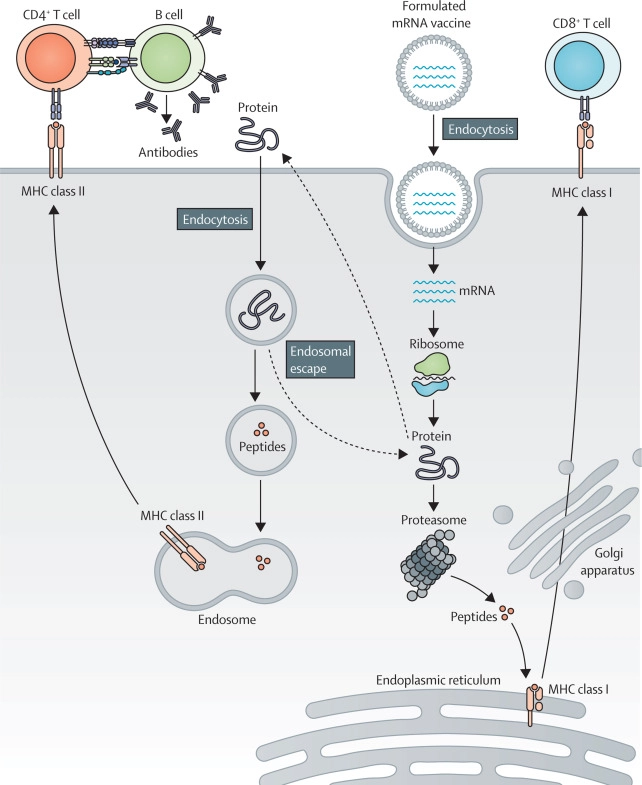
Secondly, mRNA molecules are non-infectious, and mRNA vaccines have the potential to induce both humoral and cell-mediated immunity (figure). 5 , 6
Lastly, the production of mRNA vaccines is fast and inexpensive.7
Advantages of mRNA vaccines for the treatment of cancer
- Well tolerated: adverse events are generally manageable and transient
- No genome integration: eliminates the risk of insertional mutagenesis
- Non-infectious: no pathogenic viral agents are used
- Easily degraded: reduces risk of toxicity
- Humoral and cellular immunity: necessary for activating and sustaining anti-tumour responses
- Fast and inexpensive production: laboratory-based and cell-free production
Read the whole study here.
Autor: Catherine Lund Lorentzen, MD; Prof. John B Haanen, MD PhD; Özcan Met, PhD; Prof. Inge Marie Svane, MD PhD
Published in GI-Mail 11/2022 (English edition).
- Do you already know our monthly newsletter GI-Mail with useful tips on postgraduate courses?
Sign up here. - Are you looking for vacancies or new career challenges? Here you will find the latest vacancies and job offers.
- Do you already know our monthly job-information GI-Jobs with current job offers for doctors, managers and nurses? Sign up here.
- Are you interested in up to date postgraduate courses and CME? In our education database »medicine & health« you will find new education events from over 2300 organizers.